Tetrazine Glycoconjugate for Pretargeted Positron Emission Tomography Imaging of trans-Cyclooctene-Functionalized Molecular Spherical Nucleic Acids
In this study by Auchynnikava et al., the β-cube (PET) and X-cube (CT) allowed for longitudinal follow-up of [18F]FDG-Tz in vivo, allowing to efficiently image and examine the biodistribution of [18F]FDG-Tz in mice.
Research question
In drug development, Positron emission tomography (PET) is an essential tool for investigating the pharmacokinetics of new drug candidates in early clinical studies and dose optimization studies. Pretargeted imaging in vivo in PET, together with bioorthogonal chemistry, is a recently investigated solution that enables the study of processes with slow pharmacokinetics by utilizing radiotracers with short-lived radionuclides. Recently, Radiotracers based on tetrazine ligation with trans-cyclooctene (TCO) are being studied for pretargeted PET imaging.
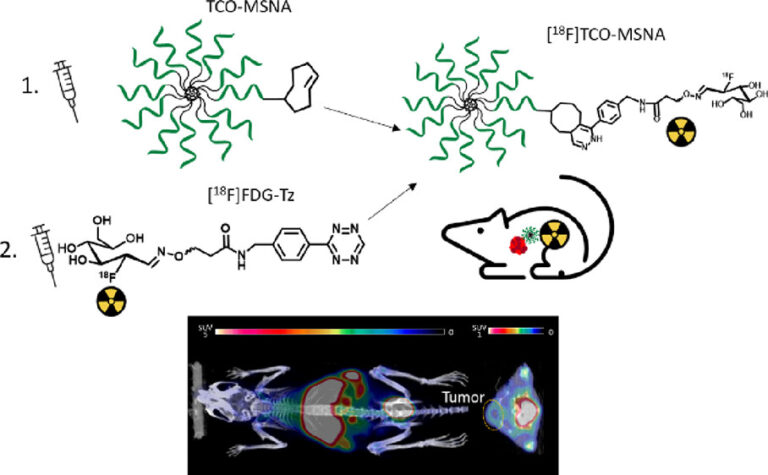
Spherical nucleic acids (SNAs)have great potential as delivery vehicles to transport a large amount of bioactive material. By functionalizing molecular spherical nucleic acids (MSNA) with TCO, an approach has been provided for tracing the MSNA biodistribution by pretargeted PET imaging based on the in vivo IEDDA reaction with tetrazine derivatives.
In this study by Auchynnikava et al., the synthesis and biological evaluation of [18F]FDG-Tz were conducted, followed by pretargeted PET imaging of TCO-functionalized MSNA in human epidermal growth factor receptor 2 (HER2)- expressing human breast cancer tumor xenograft-bearing mice.
Experiment
[18F]FDG-Tz was developed and synthesized for pretargeted PET imaging of TCO-functionalized MSNA targeting HER2 mRNA. The β-cube (PET) and X-cube (CT) were used for In Vivo [18F]FDG-Tz imaging and analysis. For in vivo [18F]FDG-Tz studies, four mice were injected with 4.0− 4.8 MBq in 50−80 μL volume via the tail vein and were imaged for 60 min. Biodistribution of [18F]FDG-Tz was evaluated in healthy mice to investigate the potential of this tracer as a pretargeted PET agent. Ex vivo evaluation occurred on organs of interest, which were dissected and weighed. Radioactivity was measured with a gamma counter. All experiments involving MSNA ex vivo measurements were performed after 60 min PET/CT imaging.
Results
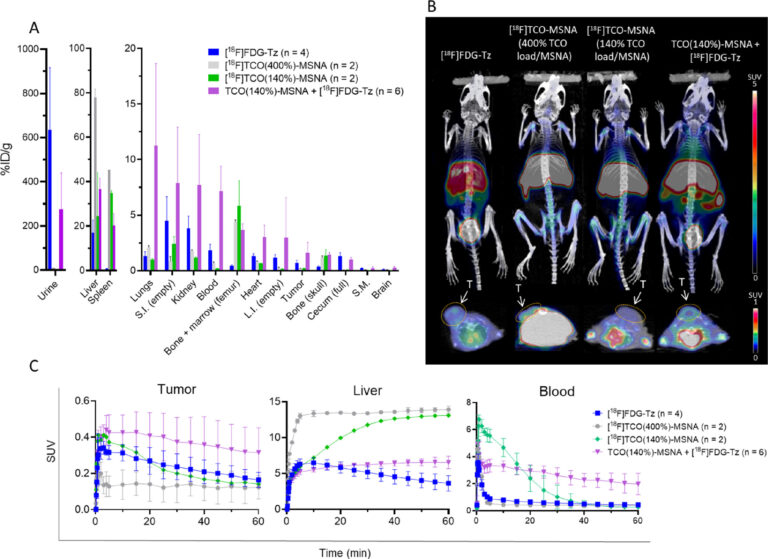
The developed radiotracer [18F]FDG-Tz showed high radiochemical purity and moderate yields. Analysis of [18F]FDG-Tz radioactivity distribution in different blood components of healthy mice showed an increase in blood cell binding and plasma protein binding. The biological evaluation indicated favorable pharmacokinetics and showed a quick elimination of [18F]FDG-Tz from blood and urinary excretion as the main elimination route. Low brain uptake (0.08 ± 0.03) at 60 min postinjection indicates low penetration through the blood-brain barrier.
Introduction of the [18F]FDG as a prosthetic radiolabeling group may cause an increase in nonspecific accumulation of the studied structure due to GLUT1 binding. The overall observed low uptake of [18F]FDG-Tz in GLUT1-rich tissues indicates the absence of GLUT1 transportation in vivo. Despite the higher observed GLUT1 transportation of [18F]FDG-Tz inside cells in vitro, no significant transportation of [18F]FDG-Tz was observed in biodistribution experiments In Vivo.
Both in vivo and ex vivo experiments showed higher radioactivity levels in the blood for the pretargeted approach, revealing higher tumor uptake for the pretargeted conditions compared to the preclicked MSNA. However, a longer lag time between TCO-MSNA and [18F]FDG-Tz injections is needed to avoid radiolabeling of the circulating TCO-MSNAs in the blood. Pretargeted experiments with TCO-functionalized MSNA indicated higher tumor uptake compared to preclicked MSNA in HER2-expressing human breast cancer xenograft-bearing mice, suggesting potential for improved cancer imaging. The potential of [18F]FDG-Tz as a pretargeted PET agent was further confirmed in a pretargeted experiment with TCO-functionalized MSNA against HER2 in tumor-bearing mice. Despite all the benefits of the pretargeting approach, new efficient PET radiotracers are still needed to adapt this multistep strategy to daily clinical use.