Imaging Metformin Efficacy as Add-On Therapy in Cells and Mouse Models of Human EGFR Glioblastoma
n this paper by Valtorta et al., the MOLECUBES β-CUBE was used to predict treatment response of Metformin in combination with Temozolomide in a glioblastoma mouse model.
Research question
Glioblastoma (GBM) is a highly aggressive tumor of the brain. Despite the efforts, response to current therapies is poor and 2-years survival rate ranges from 6-12%. Therefore, there is a continuous need for new therapies.
Experiment
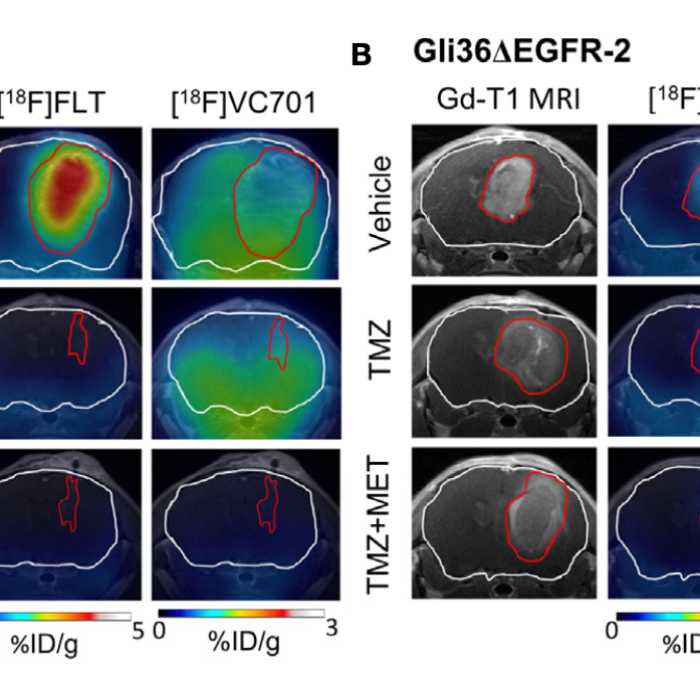
Here, we evaluated the preclinical efficacy of Metformin (MET) as add-on therapy to Temozolomide (TMZ) and the ability of [18F]FLT (activity of thymidine kinase 1 related to cell proliferation) and [18F]VC701 (translocator protein, TSPO) Positron Emission Tomography (PET) radiotracers to predict tumor response to therapy. Indeed, TSPO is expressed on the outer mitochondrial membrane of activated microglia/macrophages, tumor cells, astrocytes and endothelial cells. TMZ-sensitive (Gli36DEGFR-1 and L0627) or -resistant (Gli36DEGFR-2) GBM cell lines representative of classical molecular subtype were tested in vitro and in vivo in orthotopic mouse models. In this study, PET-MR imaging was used to evaluate therapy response (PET system: MOLECUBES β-CUBE, MR system: Bruker Biospec 70/30).
Results
The in vitro results indicate that MET increased the efficacy of TMZ on both TMZ-sensitive and on TMZ-resistant cells. In vivo results show that MET add-on significantly extended the median survival of tumor-bearing mice compared to TMZ-treated ones and reduced the rate of recurrence in the TMZ- sensitive models. PET studies with the cell proliferation radiopharmaceutical [18F]FLT performed at early time during treatment were able to distinguish between TMZ responders and non-responders, but not to predict the duration of the effect. On the contrary, [18F]VC701 uptake was reduced only in mice treated with both MET and TMZ, and levels of uptake negatively correlated with animals’ survival. Overall, the data showed that MET addition improved TMZ efficacy in preclinical glioblastoma models representative of classical molecular subtype increasing survival time and reducing tumor relapsing rate. Finally, results from PET imaging suggest that the reduction of cell proliferation represents a common mechanism of TMZ and combined treatment, whereas only the last was able to reduce TSPO. This reduction was associated with the duration of treatment response. These results suggest that TSPO-ligand may be used as a complementary molecular imaging marker to predict tumor microenvironment related treatment effects.